Research Topics in the Crooks Group
Dr. Crook's new email address: richardcrooks9830@gmail.com

The group's focus is on developing the basic science and technology that will lead to a cleaner planet and a healthier life for its inhabitants. Underpinning this basic philosophy are our core competencies in electrochemistry, catalysis, nanomaterials, and biological and chemical microsensors. Specific projects are focused in three main areas: (1) understanding the fundamental properties of well-defined mono- and multimetallic catalysts having sizes in the 1 - 2 nm range, (2) fundamentals and applications of bipolar electrodes for chem/bio sensing and for controlling ion motion in microfluidic systems, and (3) developing low cost (less than $1/assay) sensors based on the principles of origami. The most recent information about these projects can be found in the group's publications, but a brief introduction to each is provided below.
Catalysis and Nanoparticles
A central challenge in the field of catalysis is to predict effective catalyst nanostructures based on first-principles calculations. Such calculations exist for nanoparticle catalysts, but at present there are few (if any) adequate experimental models that would provide a means to test these
Low-cost Diagnostic Devices Based on the Principles of Origami
In the developed world access to medical diagnostics is relatively affordable and accessible. However, in under-resourced countries, including parts of Africa, Asia, and South America, such tests are not available. We are addressing this need by developing the basic science and technology necessary for fabricating low-cost diagnostic sensors using simple materials, like paper, and simple fabrication techniques like laser-jet printing and origami (Japanese paper folding). The objective is to develop multiplexed sensors for disease detection and immunization status that are very inexpensive (10 cents to 1 dollar), easy to use (colorimetric detection via the naked eye), and that require little or no power to operate.

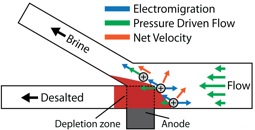
Electrochemically Mediated Desalination
The world is facing a global challenge to reliably supply its population with safe water due to shortages stemming from population growth, climate change, contamination of available fresh water supplies, and public policy. Therefore, it is increasingly likely that a variety of desalination technologies will be required to supplement natural fresh water reserves. Although technologies exist to desalinate water, vast amounts of energy consumption and therefore costs are associated with these processes. Consequently, the challenge we are working to solve is to develop an energy-efficient desalination technology. The current benchmark for seawater desalination is a membrane based technique called reverse osmosis (RO). However, RO has plateaued in terms of energy efficiency and suffers from several limitations stemming from the membrane. Our aim is to solve these problems through the development of a fundamentally new desalination process that doesn't require a membrane, but rather that takes advantage of the naturally abundant Cl- concentration in seawater to generate a local electric field strength capable of separating ions. Our approach, which we call electrochemically mediated desalination (EMD), is illustrated in the scheme and also described in a recent publication. A seawater feed is separated into brine and desalted water streams at the junction of a branched microchannel where an anode is present. Here, Cl- ions are oxidized to Cl2, therefore producing an ion depletion zone. Due to the increased resistivity in the localized ion depletion zone, a large proportion of the applied potential bias drops in this region to generate an electric field gradient that separates ions to the brine channel, thus producing desalted water. We have operated these devices at an energy efficiency of 25 mWhL-1 (25 ± 5% salt rejection, 50% recovery), which is near the theoretical minimum amount of energy required for this process (~17 mWhL-1). Beyond the energy efficiency achieved by EMD, we are excited about the possible benefits arising from the elimination of a membrane from the desalination process. Moreover, our approach requires only a simple power supply to operate and therefore, in the future, may be employed in resource-limited settings with a battery or low power, renewable energy source. Our goal is to further elucidate the operating principles of this desalination technology to optimize and scale its use. This research is supported by the Department of Energy and our industrial partner, Okeanos Technologies.
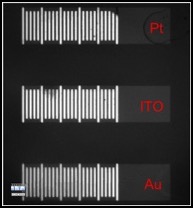
Bipolar Electrodes
Most commercially viable sensors are based on electrochemical detection, but they usually make use of just a single electrode and hence can only sense a single analyte. The goal of our work with bipolar electrodes (BPEs) is to improve on this paradigm by developing the theory, experimental approach, and engineering technology that will lead to large-scale arrays of electrodes that are easy to fabricate and control, inexpensive, and that require low power. This project has resulted in arrays of up to 1000 electrodes that are controlled with a single, simple power supply, and methods for preconcentrating analytes by nearly 1,000,000-fold. A recent review article summarizes much of our recent work with BPEs, which is financially supported by the Department of Energy and the Defense Threat Reduction Agency (DTRA).
A new project that we are very excited about involves rapid screening of electrocatalystsusing BPEs. The current focus of these experiments is on catalyst candidates for the oxygen reduction reaction (ORR), but it is applicable to any electrocatalytic reaction. The BPE configuration used for screening is shown in the figure (this is a movie: click to play it). It consists of three BPEs embedded within a microfluidic channel. An important aspect of this device is that no direct electrical connection to the electrodes is required, which means that large arrays of catalysts can be screened using a single, simple power supply. When catalyst candidates are present on the cathodic poles of the BPEs (right side: Pt, Indium-doped SnO2, and Au in this case), they can drive the reduction of oxygen. This reduction reaction is electrically coupled to the oxidation of Ag microbands present on the anodic poles. The best catalyst (Pt in this case) results in rapid dissolution of most of the Ag microbands, signaling that it is the best catalyst. By measuring the rate of dissolution of the Ag microbands, the kinetics of the electrochemical reaction can be determined. Moreover, because each device may consist of thousands of BPEs, large numbers of catalysts can be quantitatively evaluated in just a few minutes. We hope that this approach will result in better catalytic materials, and hence lower energy requirements for carrying out chemical reactions, and technologies that rely on clean alternatives to hydrocarbon fuels. More information about this project is available in a recent publication.
Graduate Student Nevena Ostojic had the recent honor of having her article “Electrocatalytic Reduction of Oxygen on Platinum Nanoparticles in the Presence and Absence of Interactions with the Electrode Surface” published as part of the ACS Editors' Choice initiative! You can see the article and presentation here: http://pubs.acs.org/doi/full/10.1021/acs.langmuir.6b02578